-
About
-
Our Brand
-
Products
-
Community
Community
Blog
Blog
The history of steel - 1
- Writer
- STEELTOPIA
- Date
- 23-08-30
The steel industry has evolved from ancient times, where a few men operated small furnaces producing 10kg periodically, to modern integrated steel mills producing approximately 1 million tons of steel annually. In 1987, Nippon Steel, the largest commercial steel company in Japan, was responsible for producing 26 million tons, with over 10 major companies worldwide producing over 10 million tons each. Excluding non-industrialized countries without employment data, approximately 1.7 million people were employed in 1987, producing 430 million tons of steel. This equates to producing about 250 tons of steel per person annually, showcasing an extremely utiliztion of human effort.
The earliest steel production
Iron production bean in Anatolia around 2000 BC, and the Iron Age was established arouond 1000 BC. Iron smelting technology spread widely, reaching the westernmost part of Europe around 500 BC and reaching China by 400 BC. Iron ore was widely distributed, and another raw material, charcoal, was also readily available.
The carbon content of early iron varied from very low (0.07%) to high (0.8%), with the latter consituting true steel. When the carbon content of steel is above 0.3%, if it is quenched at temperatures around 850℃~900℃ (1,550°F~1,650°F), the material becomes very hard but also brittle. Ductility can be reduced by reheating the steel within the range of 350°C~500°C(660°F~930°F) in a process known as tempering. This type of heat treatment was introduced to the Egyptians around 900 BC (as inferred from the microstructure of remaining artifacts), laying the foundation for the steel industry that produced an ideal material for the production of swords and blades.
Chinese quickly transitioned from producing low-carbon iron to high-carbon cast iron, and evidence suggests that they were able to produce heat-treated steel during the early Han Dynasty (206 BC~AD 25).
Romans, who were considered organizers rather than innovators, played a crucial role in disseminating ironmaking knowledge, leading to a signigicant increase in pig iron production across the Roman world.
As the influence of Rome declined, the iron industry in Europe remained unchanged, much like it had been before, and the rest of the world showed no significant changes for centuries.However, in the early 15th century, water power began to be used to inject air into furnaces. As a result, the temperature of the furnace increased to over 1,200°C (2,200°F), leading to the formation of a carbon-rich liquid, known as cast iron, instead of producing solid iron blooms. To reduce its carbon content and create wrought iron, the solidified cast iron was melted in a finely controlled process using charcoal as fuel in an oxidizing atmosphere.This process removed carbon and yielded a semi-solid bloom, which was then shaped through hammering after cooling. To convert pig iron into steel and increase its carbon content, the carburizing process was employed. Iron billets, comprising around 10 to 14 tons of metal and about 2 tons of charcoal, were heated with charcoal in a sealed crucible placed inside a large, bottle-shaped furnace. As the furnace heated up, carbon released from the charcoal diffused into the iron. To achieve uniformity, the initial product was taken out of the furnace, forged, and then reheated in the furnace with charcoal. During this reheating process, carbon monoxide gas was internally formed from non-metallic impurities. As a result, oxide scale formed on the surface of the steel detaches from the base metal and swells, leading to the use of the term "blister steel" to describe the product. This process spread throughout Europe as the method for producing top-quality blister steel from Swedish iron. One of the common steel products produced using this method was weaponry. Crafting a fine sword, for instance, required about 20 repetitions of the hammering, and carburizing process, followed by final refinement and tempering, making the material quite expensive.
Crucible Steel
In 1751, a significant advancement took place when Benjamin Huntsman established a steelworks in Sheffield, England. Here, steel was produced by melting blister steel in clay crucibles using coke as fuel at temperatures of 1,500°C to 1,600°C (2,700°F to 2,900°F). Originally, the weight of a blister was about 6 kilograms, but by 1870, it had increased to 30 kilograms. The weight of the blister was limited to around 10 kilograms, the maximum that a man could handle from the hot furnace. The molten metal was cast into ingots with a square cross-section of approximately 75mm and a length of 500mm, although various other castings were also made.
Sheffield became a hub for crucible steel production. During its peak in 1873, production reached 110,000 tons, about half of the world's production. The crucible process spread to Sweden and France after the Napoleonic Wars, and in Germany, it became associated with Alfred Krupp in Essen. In 1895, a small crucible steel plant began in Tokyo, and crucible steel was produced in the United States from around 1860 in Pittsburgh, Pennsylvania, using pig and wrought iron feedstock.
The crucible process allowed for the addition of alloying elements to the crucible's molten metal, enabling the production of alloy steels for the first time. However, it declined from the early 20th century as electric furnaces became more prevalent. The last known working crucible furnace in Sheffield operated until 1968.
The Bessemer Steel process
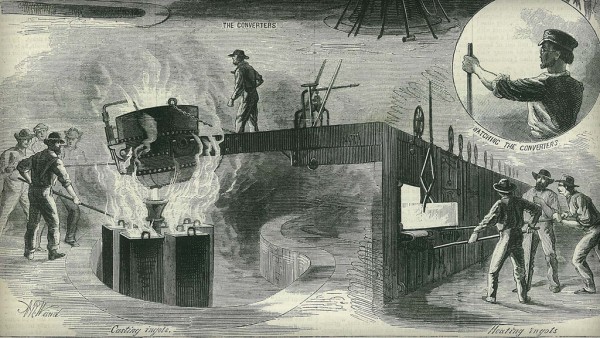
Mass steel production became feasible in 1855 when Henry Bessemer obtained a British patent for the pneumatic steelmaking process. A similar approach was reportedly employed in the United States by William Kelly in 1851, although it wasn't patented until 1857. Bessemer utilized a ship-shaped vessel lined with ganister, a refractory material infused with air-entrained silica. Air was blown upwards through molten pig iron in the vessel. Bessemer recognized that the subsequent oxidation of silicon and carbon in the iron released heat. With the use of a sufficiently large vessel, this heat production could offset the heat loss. Consequently, a temperature of 1,650°C (3,000°F) could be achieved within a blowing period of 15 minutes, with the charge weighing around 0.5 tons.
However, one challenge with the Bessemer process was its restriction to low-phosphorus and low-sulfur pig iron. The addition of basic fluxes, such as lime, could remove these elements. Yet, the resultant basic slag would have deteriorated the acid refractory lining of the Bessemer converter. In Britain, there was an abundant supply of low-phosphorus iron ore, mainly hematite. Meanwhile, in the United States, phosphorus was more costly than richer ores.
In 1878, Sidney Gilchrist Thomas and Percy Gilchrist introduced an advancement – a basic-lined converter utilizing calcified dolomite as a refractory material. This innovation enabled the use of lime-rich slag, which could retain phosphorus and sulfur in solution. This "basic Bessemer" process wasn't widely adopted in Britain and the United States. However, it harnessed phosphate rock from Alsace and Lorraine, serving as a foundation for the steel industries' growth in Belgium, France, and Germany.
As a result of these advancements, by the year 1900, global steel production had escalated to approximately 50 million tons. This extraordinary growth marked a substantial milestone in the history of steel manufacturing.
Open hearth furnace
The alternative steelmaking process was developed in the 1860s by William and Friedrich Siemens of the United Kingdom, as well as Pierre and Émile Martin of France. In this process, a regenerative chamber lined with refractory material containing nonflammable substances was used. Air and preheated fuel gases at 800°C were combusted within the chamber. This resulted in a flame temperature of around 2,000°C, which was sufficient to generate ample heat for melting the charge.
During the refining stage, a reaction occurred between slag (which had iron ore added) and the molten metal to remove carbon, manganese, and silicon from the metal. While an initial charge of 10 tons was used, the furnace's capacity gradually increased to 100 tons and eventually reached 300 tons. While initially acid-treated furnaces were employed, a fundamental process that effectively removed phosphorus and sulfur from the charge was later developed. This process generated heat over a span of 12 to 18 hours, allowing ample time for material analysis and composition adjustment before extraction from the furnace.
The primary advantage of open-hearth furnaces was their flexibility. The charge could consist entirely of molten pig iron, entirely of cold scrap, or a combination of the two. As a result, steel was able to move away from relying solely on liquid iron as its source.
By 1950, 90% of the steel production in the United Kingdom and the United States was carried out using the open-hearth process. Additionally, even as late as 1988, Eastern European countries were producing over 96 million tons annually using this method.
Oxygen steelmaking
In the existing open-hearth process, a significant amount of time-consuming reaction between slag and metal was necessary for steel refinement. After World War II, the availability of oxygen tonnage enabled various attempts to accelerate the steelmaking process by directly injecting oxygen into the charge. The Linz-Donawitz (LD) process, developed in Austria in 1949, involved blowing oxygen through a window at the top of a vessel shaped similarly to the Bessemer converter. Absence of cooling effects from inert nitrogen gas in the air allowed the retained heat in the off-gas to be utilized for melting added scrap in the molten pig iron. Additionally, the process facilitated the production of basic slag by adding lime to the charge, effectively removing impurities like sulfur and phosphorus. Known as the Basic Oxygen Process (BOP), this method enabled the production of 200 tons of steel within a Tap-to-Tap time of 60 minutes from a charge composed of up to 35% scrap. The original charge size of the basic oxygen furnace increased to 400 tons, and with lower silicon content, the tapping time was reduced from 15 to 20 minutes.
Shortly after the introduction of the LD process, a modification involving injecting burnt lime along with oxygen through a lance was developed. This process, known as LD-AC (named after ARBED steel company in Luxembourg and Centre National in Belgium) or OLP (Oxygen Lime Powder) process, further improved the effective refinement of pig iron derived from European ores with higher iron content. A resurgence of the basic Bessemer concept was developed in the mid-1960s in Canada and Germany. This process utilized two concentric tuyeres, with hydrocarbon gas in the outer annulus and oxygen in the center. Originally known by the German acronym OBM (Oxygen bodenblasen Maxhuette, "oxygen bottom blowing Maxhuette"), this product was recognized as Q-BOP in North America. Starting around the 1960s, all oxygen steelmaking processes replaced open-hearth and Bessemer processes on both sides of the Atlantic.
Steelmaking in Electric Arc Furnace
With the development of the power industry until the late 19th century, the idea of using electricity as an energy source for steel production emerged. By 1900, small electric furnaces capable of melting about 1 ton of steel were introduced. These electric furnaces were mainly used for producing tool steel and replaced the traditional method of smelting in a hearth. By 1920, the size of the electric furnaces had increased to a capacity of 30 tons. Power supply was provided by a 3-phase 7.5 megavolt-ampere system, with three graphite electrodes being supplied through the roof, forming an arc between the electrodes and the furnace.
By 1950, the capacity of the electric furnaces had further increased to 50 tons, and the power supply reached 20 megavolt-amperes. Smaller arc furnaces were filled with acid refractories and were mainly used as melting units due to limited refining. Larger furnaces, however, had a basic lining made of refractory material and incorporated limestone to form slag. This slag helped remove impurities such as silicon, sulfur, and phosphorus from the molten metal. The furnaces could operate with a charge of either all scrap or a mixture of scrap and pig iron, producing steel of excellent quality with low sulfur and phosphorus content (0.01%). As a result, the basic electric arc process proved ideal for producing low-alloy steels and by 1950 had nearly replaced the open-hearth process of this capacity. During that time, electric furnaces produced about 10% (roughly 200 million tons worldwide) of the global steel production. However, the introduction of oxygen into the basic arc process accelerated its efficiency, causing electric furnaces to account for almost 30% of steel production by 1989. In that year, global steel production amounted to 770 million tons.
The information provided on this webpage is intended solely for informational purposes. Steeltopia does not make any explicit or implied representations or warranties regarding the accuracy, comprehensiveness, or validity of this information.


 HOME
HOME